-
PDF
- Split View
-
Views
-
Cite
Cite
Jose Perea, Anton Garcia, Gustavo Gómez, Raquel Acero, Francisco Peña, Sara Gómez, Effect of light and substratum structural complexity on microhabitat selection by the snail Helix aspersa müller, Journal of Molluscan Studies, Volume 73, Issue 1, February 2007, Pages 39–43, https://doi.org/10.1093/mollus/eyl031
Close - Share Icon Share
ABSTRACT
Terrestrial snails choose their microhabitat according to a number of environmental factors. We evaluated the effect of light intensity and substratum complexity on microhabitat preference of the terrestrial snail Helix aspersa using a multi-factorial design. The snails were offered two levels of light intensity and two types of structural complexity, hence 16 treatments in total were used: 12 in which choice was offered and 4 in which no choice was offered. The snails preferred ambient light over dim light, regardless of substratum complexity, and complex over smooth substrata, regardless of light intensity. The level of one factor did not affect the response to the other. Thus, the results revealed a preference for microhabitats with greater light intensity and that were structurally complex, and a rejection of dimly lit microhabitats with smooth substrates.
INTRODUCTION
The small-scale distribution of mobile animals is typically determined by a number of biotic and abiotic components in their microhabitat, some of which have more influence than others (Pennings et al., 1998). Biotic components include the density of conspecific or interspecific competitors, prey and potential predators (Martin & Salvador, 1995). Abiotic components such as substratum structural complexity and light intensity are important for invertebrates (Hill et al., 2004). Both factors affect the vulnerability of the animals in relation to predators and environmental stress (Lima & Dill, 1990; McNett & Rypstra, 2000).
The common pattern of microhabitat choice among invertebrates, especially gastropods, is the preference for protective microhabitats (Greenstone, 1984; Jones & Boulding, 1999). However, their behaviour in relation to light intensity is controversial; in the marine context, intertidal slugs display a strong preference for darkness (Barbeau, Durelle & Aiken, 2004), but certain intertidal snails display positive phototropism when they are under water and negative phototropism when out of the water (Meadows & Campbell, 1972). Although these two factors are likely to influence habitat choice of terrestrial snails, little is currently known about their effect.
Habitat selection and nonrandom spatial distribution patterns have been described for some terrestrial snail populations (Lind, 1988, 1989; Attia et al., 1997; Willig, Sandlin & Gannon, 1998). The heterogeneity of spatial distribution has been linked to availability of certain plant species, light intensity or degree of humidity. On a small scale, the terrestrial snail Theba pisana responds to light and wind direction, avoiding the most exposed microhabitats (Cowie, 1985). Helix aspersa survives over a wide latitude range, including dry conditions and extreme temperatures (Chevallier, 1977; Iglesias, Santos & Castillejo, 1996); Habitat selection has been shown to affect growth, survival and fecundity (Cowie, 1985; Dyer & Landis, 1996); consequently, unravelling the factors involved in microhabitat selection is crucial for the improvement of snail rearing systems.
The aim of this study was to assess the importance of structural complexity of substratum and light intensity on microhabitat selection by H. aspersa.
MATERIAL AND METHODS
Collection of animals and rearing conditions
Adult snails were collected in April 2005 from the outdoor parks at the CIFA experimental farm (Andalusian Agrarian Research and Training Centre, Hinojosa del Duque, Córdoba, Spain) when they were 15 weeks old and had an average diameter of 30 ± 2.5 mm. The animals were transported to the Snail Laboratory at the University of Cordoba and were kept in experimental conditions that allow natural behaviour (García et al., 2006), and minimize possible distortions in behavioural responses and alterations in their microhabitat selection process.
The snails were maintained under artificial light (14:10 h light/dark cycle), in a dark room illuminated by fluorescent lights (cool white light without ultraviolet component). The use of ventilation and a humidifier regulated the temperature and the relative humidity of the laboratory. During the day, the mean relative humidity and temperature was 63% and 25°C, respectively. During the night, mean relative humidity rose to 77% and temperature decreased to 19°C. In these conditions, snails rested during the day and were actively moving and foraging at night. Leftover food was removed and the boxes were cleaned when the laboratory lights were turned on (8:00 a.m.). After 14 hours, at 10:00 p.m., snails were activated by switching off the laboratory lights and spraying the container with water.
Snails had one week of adaptation to the experimental conditions in a plastic container (100 × 100 × 8 cm), prior to the start of the experiment. During the adaptation week, the location of the snails during the resting period was recorded three times a day, at 8:00 a.m., 3:00 p.m. and 9:00 p.m. The animals did not significantly modify their location during the day and therefore it was decided that only one score per day, half way through the resting period, should be carried out.
Laboratory experiment
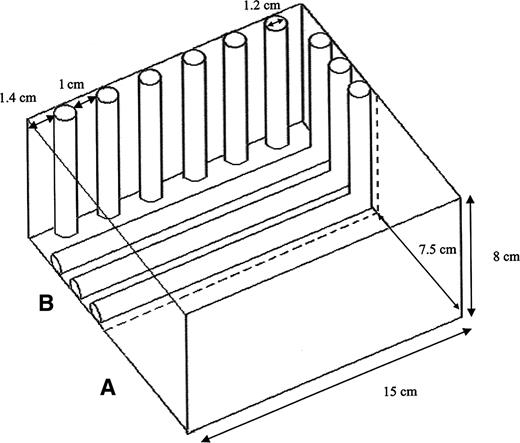
The effects of two levels of light and two levels of structural complexity were studied. The light intensity reaching the snails was either ambient lighting (7.60 µmol photon cm−2 s−1) or a lower level achieved by covering all walls on one half of each box with opaque plastic sheets (2.55 µmol photon cm−2 s−1). For the structurally complex areas, translucent polyethylene tubes 1.2 cm in diameter were placed in the boxes, 1 cm apart (Fig. 1). For the structurally smooth areas, the surface of the box was not modified.
Scheme of the experimental box for substratum structural complexity. A. Smooth B. Complex.
The trials were conducted in 15 × 15 × 8 cm translucent plastic boxes, divided into two symmetrical halves, without a physical separation, and each one with a different combination of light exposure and structural complexity. The experiment was designed to be factorial and orthogonal, following Barbeau et al. (2004), enabling the effect of each factor to be studied individually as well as the interactions between both factors. In order to obtain independent data, only the information from one of the halves was recorded and the alternative combination of data was discarded.
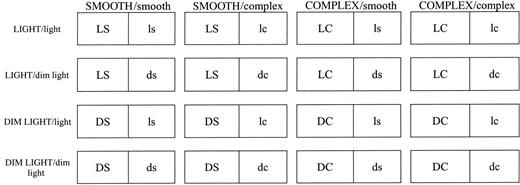
Each of the four possible combinations of light and substratum conditions was represented on one side of each box and paired with each of the four combinations on the other side. In all permutations, a total of 16 combinations were allowed: 12 where a choice was offered and 4 without a choice, as shown in Figure 2. Only the observations on the left half of the box were recorded (marked with upper-case letters in Fig. 2); the alternative combination, in the right half of the box, was ignored (marked with lower-case letters).
Diagram of the 16 treatment combinations in the multi-factorial choice experiment. In a treatment combination, 10 snails were offered a choice between two halves of the experimental box, each with a particular light intensity and structural complexity of substratum. To obtain independent data, only one half of the rearing box, indicated by the uppercase letters, was included in the data analysis. Abbreviations: L or l, light; D or d, dim light; S or s, smooth and C or c, complex.
A sample of 160 snails was randomly distributed in these 16 experimental groups. At the start of the experiment 10 snails were placed on a line across the centre of each box. Each half of the box had a surface area of 465 cm2; therefore, if all the animals made the same choice and chose the same half of a rearing box, the density would be less than 0.02 animals/cm2. This density is less than that proposed by Dupont-Nivet et al. (2000) and Mayoral et al. (2004), and presumably does not condition their choice.
Measurements and statistical analysis
The experiment was conducted on 35 days within the period between 18 April and 22 May 2005. Observations were made daily at 3:00 p.m. on each of the 16 experimental boxes. The number of snails occupying the half of the box marked with upper-case letters was recorded (Fig. 2).
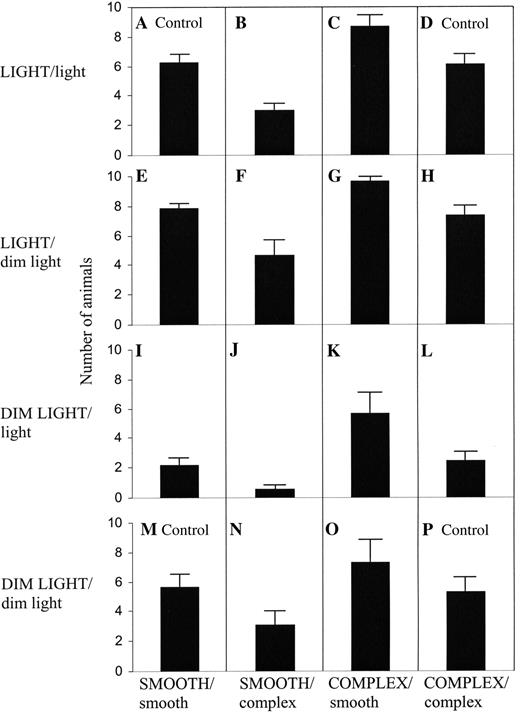
The selection of microhabitat was analysed using a two-way ANOVA. Each of the factors contains two levels and four combinations (two with a choice and two without a choice): light intensity: LIGHT/light, LIGHT/dim light, DIM LIGHT/light, DIM LIGHT/dim light; and structural complexity: SMOOTH/smooth, SMOOTH/complex, COMPLEX/smooth, COMPLEX/complex. Finally, in order to evaluate preference in each combination individually, a one-way ANOVA was applied along with an SNK multiple range test. All statistical procedures were performed using SPSS software (SPSS Inc., version 11.5).
RESULTS
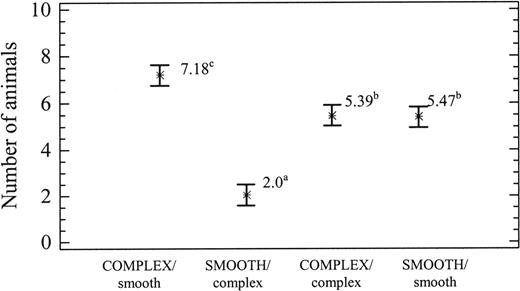
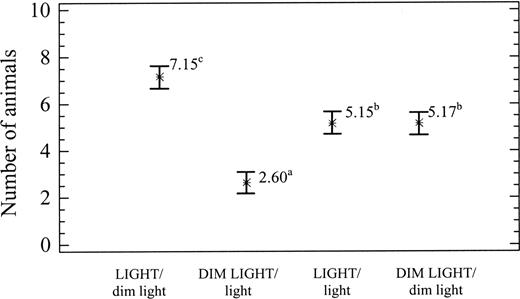
Table 1 shows the results of the two-way analysis of variance. Highly significant differences were observed in relation to light intensity (P < 0.0001) and structural complexity (P < 0.0001); but there was no significant interaction between light and structural complexity (P > 0.05). Using the SNK homogeneity text, three distribution groups were established in relation to light intensity and structural complexity, as shown in Figures 3, 4, respectively. Figure 3 shows that the snails displayed a preference for the complex substratum over the smooth substratum, and Figure 4 shows a preference for light over dim light.
Average occupancy (mean ± SE) in the four combinations of substratum structural complexity. Superscript letters indicate significant differences between means.
Average occupancy (mean ± SE) in the four combinations of light intensity. Superscript letters indicate significant differences between means.
ANOVA results for the number of snails in the observed half of a experimental box (with a particular light intensity and structural complexity) during the multi-factorial choice experiment.
| Source of variation . | df . | Mean square . | F-ratio . | P-value . | Multiple comparisons . |
|---|---|---|---|---|---|
| Lighting | 3 | 330.90 | 44.89 | 0.00001 | |
| Complexity | 3 | 439.11 | 59.57 | 0.00001 | |
| Lighting × Complexity | 9 | 11.20 | 1.52 | 0.1392 | Ll: Sca Ccb Ssb Csc |
| Ld: Sca Ccb Ssb Csc | |||||
| Dl: Sca Ccb Ssb Csc | |||||
| Dd: Sca Ccb Ssb Csc | |||||
| Ss: Dla Ddb Llb Ldc | |||||
| Sc: Dla Ddb Llb Ldc | |||||
| Cs: Dla Ddb Llb Ldc | |||||
| Cc: Dla Ddb Llb Ldc | |||||
| Error | 2683.3 | 7.37 |
| Source of variation . | df . | Mean square . | F-ratio . | P-value . | Multiple comparisons . |
|---|---|---|---|---|---|
| Lighting | 3 | 330.90 | 44.89 | 0.00001 | |
| Complexity | 3 | 439.11 | 59.57 | 0.00001 | |
| Lighting × Complexity | 9 | 11.20 | 1.52 | 0.1392 | Ll: Sca Ccb Ssb Csc |
| Ld: Sca Ccb Ssb Csc | |||||
| Dl: Sca Ccb Ssb Csc | |||||
| Dd: Sca Ccb Ssb Csc | |||||
| Ss: Dla Ddb Llb Ldc | |||||
| Sc: Dla Ddb Llb Ldc | |||||
| Cs: Dla Ddb Llb Ldc | |||||
| Cc: Dla Ddb Llb Ldc | |||||
| Error | 2683.3 | 7.37 |
Unplanned comparisons (SNK test) were done for the number of animals in the observed half of the experimental box. Letters next to the treatments indicate significant differences between the means, ‘a’ being the lowest value and ‘c’ the highest.
Abbreviations: The treatments are abbreviated as follows, for light intensities: Ll, LIGHT/light; Ld, LIGHT/dim light; Dl, DIM LIGHT/light; Dd, DIM LIGHT/dim light; for structural complexity of substratum: Ss, SMOOTH/smooth; Sc, SMOOTH/complex; Cs, COMPLEX/smooth; Cc, COMPLEX/complex (see Fig. 2).
ANOVA results for the number of snails in the observed half of a experimental box (with a particular light intensity and structural complexity) during the multi-factorial choice experiment.
| Source of variation . | df . | Mean square . | F-ratio . | P-value . | Multiple comparisons . |
|---|---|---|---|---|---|
| Lighting | 3 | 330.90 | 44.89 | 0.00001 | |
| Complexity | 3 | 439.11 | 59.57 | 0.00001 | |
| Lighting × Complexity | 9 | 11.20 | 1.52 | 0.1392 | Ll: Sca Ccb Ssb Csc |
| Ld: Sca Ccb Ssb Csc | |||||
| Dl: Sca Ccb Ssb Csc | |||||
| Dd: Sca Ccb Ssb Csc | |||||
| Ss: Dla Ddb Llb Ldc | |||||
| Sc: Dla Ddb Llb Ldc | |||||
| Cs: Dla Ddb Llb Ldc | |||||
| Cc: Dla Ddb Llb Ldc | |||||
| Error | 2683.3 | 7.37 |
| Source of variation . | df . | Mean square . | F-ratio . | P-value . | Multiple comparisons . |
|---|---|---|---|---|---|
| Lighting | 3 | 330.90 | 44.89 | 0.00001 | |
| Complexity | 3 | 439.11 | 59.57 | 0.00001 | |
| Lighting × Complexity | 9 | 11.20 | 1.52 | 0.1392 | Ll: Sca Ccb Ssb Csc |
| Ld: Sca Ccb Ssb Csc | |||||
| Dl: Sca Ccb Ssb Csc | |||||
| Dd: Sca Ccb Ssb Csc | |||||
| Ss: Dla Ddb Llb Ldc | |||||
| Sc: Dla Ddb Llb Ldc | |||||
| Cs: Dla Ddb Llb Ldc | |||||
| Cc: Dla Ddb Llb Ldc | |||||
| Error | 2683.3 | 7.37 |
Unplanned comparisons (SNK test) were done for the number of animals in the observed half of the experimental box. Letters next to the treatments indicate significant differences between the means, ‘a’ being the lowest value and ‘c’ the highest.
Abbreviations: The treatments are abbreviated as follows, for light intensities: Ll, LIGHT/light; Ld, LIGHT/dim light; Dl, DIM LIGHT/light; Dd, DIM LIGHT/dim light; for structural complexity of substratum: Ss, SMOOTH/smooth; Sc, SMOOTH/complex; Cs, COMPLEX/smooth; Cc, COMPLEX/complex (see Fig. 2).
Secondly, light intensity and structural complexity were analysed individually using a one-way ANOVA (Table 1, Fig. 5). In the treatment combinations with uniform conditions of structural complexity and light intensity (Fig. 5 A, D, M, P) snails did not have preference for either half of the box. We observed that, when the same combination of light was offered in both halves of the box, either LIGHT/light or DIM LIGHT/dim light, the animals showed a preference for complex substratum (Csc) and rejected smooth substratum (Sca). Furthermore, in identical conditions of structural complexity (COMPLEX/complex and SMOOTH/smooth), the animals displayed a preference for light (Ldc), rejecting dim light (Dla).
Number of Helix aspersa snails (mean ± SE) in the 16 different combinations of light intensity and structural complexity.
By comparing the choice between a smooth dimly lit area and a light complex area (Fig. 5 G, J), we observed a strong rejection of the smooth dimly lit area and a strong preference for the light complex area. Furthermore, when the choice was offered between a smooth light area and a dimly lit complex area (Fig. 5F, K), the snails did not display either preference or rejection for either half of the boxes. Therefore, both factors have a similar influence on the choice of microhabitat of Helix aspersa snails.
DISCUSSION
In this study of microhabitat selection, snails preferred ambient light over dim light, regardless of the structural complexity of the substratum. They also displayed a preference for a complex substratum over smooth, regardless of the light intensity. Furthermore, their strongest preference was for the halves of boxes that combined the two conditions of complex substratum and light (Fig. 5G).
The tendency to opt for a certain microhabitat could be due to active or passive selection, but only active selection is indicative of preference (Crowe & Underwood 1998). Active selection occurs when a mobile organism selects a particular microhabitat more frequently than others. Passive selection occurs when the observed preference is due to the inability to choose another microhabitat; i.e. movement may be more difficult and slower on one kind of microhabitat than another (Olabarria, Underwood & Chapman, 2002). In this experiment, evidence of active selection was observed, since the experimental design allowed us to confirm preference or rejection by comparing choice situations to no-choice situations. In addition, the results obtained in no-choice treatments discount the incidence of other factors on microhabitat selection, like aggregation behaviour.
Generally, the interactions between the environmental factors of a microhabitat have greater importance than variations in each individual factor when it comes to explaining spatial distribution patterns of populations (Meadows & Campbell, 1972); however, in the present study, no interactions were registered between light and substratum complexity.
For terrestrial snails, there are no studies that simultaneously examine the effect of light and substratum complexity on microhabitat selection. However, the behavioural responses of certain gastropods and other invertebrates when faced with some of these situations have been described (Garrity, 1984; Cowie, 1985; Jones & Boulding, 1999; Hill et al., 2004).
Experiments that evaluate the effect of structural complexity on the behavioural responses of intertidal gastropods usually analyse the topography, type or texture of the substratum. Intertidal gastropods show a preference for high complexity of substratum (Littorina sitkana, Jones & Boulding, 1999; Tegula funebralis, Marcheti & Geller, 1987; Colisella digitalis, Gallien, 1985 and Morula marginalba, Moran, 1985). Barbeau et al. (2004) demonstrated that the intertidal slug Onchidoris bilamellata prefers rough substrata, but only when they were in dark conditions. Terrestrial arthropods commonly prefer complex structures (e.g. spiders, Greenstone, 1984; beetles, Crist et al., 1992; spider Argiope trifasciata, McNett & Rypstra, 2000; praying mantid Ciulfina sp., Hill et al., 2004). Generally, the preference for complex substrata is associated with foraging efficiency (Hill et al., 2004), reduction of predation risk (Lima & Dill, 1990) and protection against environmental stress (Marcheti & Geller, 1987; Jones & Boulding, 1999). In terrestrial snails like Helix aspersa, alleviation of environmental stress may be a key factor in microhabitat selection.
Though terrestrial snails are very sensitive to dehydration, they have several strategies to cope with thermal and desiccation stress, like secretion of an epiphragm, clustering behaviour, selective compartmentalization of body fluids and metabolic depression (Arad & Avivi, 1998; Ansart, Vermon & Daguzan, 2002).
The choice of protective microhabitats is another mechanism to reduce thermal and desiccation stress. Selection of less stressful microhabitats has been reported as a behavioural adaptation used by many species of intertidal snails to control their internal temperature and water loss (Garrity, 1984; Gallien, 1985; Moran, 1985). It has been reported that some terrestrial snails respond to environmental conditions, by avoiding warmer and dryer areas (e.g. Theba pisana, Cowie, 1985; Caracolus caracolla, Willig et al., 1998). The present study provides evidence that H. aspersa prefer complex substrata and this preference could be explained by a mechanism to combat physical stress. Complex substrata retain more moisture than smooth ones by reducing water loss from evaporation and wind (Monteith & Unsworth, 1990; Helmuth, 1998). Furthermore, complex substrata show reduced temperature oscillation (Williams & Morritt, 1995; Jones & Boulding, 1999).
Another aspect to be considered is the vulnerability of snails to predators. Slow-moving animals tend to camouflage themselves in their environment, whereas fast-moving animals tend to run away. Complex substrata can diminish visibility and, thus, the predation ratio for terrestrial snails, as it does for other slow-moving invertebrates (Lima & Dill, 1990; Elkin & Baker, 2000).
In relation to light intensity, there is no common pattern among gastropods. Intertidal slugs display photonegative behaviour (Barbeau et al. (2004)), while some species of freshwater snails prefer to feed in strongly illuminated areas, leading to a reduction of population density in shaded areas (Thomas & Daldorph, 1991). These findings were mainly explained by food quality differences between illuminated and shaded areas (Li et al., 2005).
For terrestrial snails, it has been demonstrated that they respond to light by avoiding direct sunlit areas. This behavioural response has been linked to desiccation and thermal stress (Cowie, 1985). In this study, snails preferred fully lit areas over dimly lit ones. However, the light used did not result in an increase in temperature or desiccation.
The preference for fully lit areas revealed in this study could be explained by foraging efficiency. It is well know that the diet of H. aspersa is based on living plants and grasses (Iglesias & Castillejo, 1999; Chevalier et al., 2000). In field and outdoor rearing parks H. aspersa often rests on vegetation indirectly illuminated, partially shaded by other vegetative structures (Perea, 2004). However, the species does not normally rest in full sun, and this behavioural response needs further study. A modification of the response of H. aspersa in relation to light intensity would be expected if the intensity and wavelength used in the experiment were modified (for example using ultraviolet light).
It is possible that the daily rhythms and behavioural responses of H. aspersa were distorted in this laboratory experiments by handling stress (Underwood, Chapman & Crowe, 2004). However, in this experiment alteration in the snails' behaviour was minimized by following the laboratory handling system proposed by García et al. (2006). This system favours the welfare of the snails by avoiding direct handling, as indicated by Perea, 2004. In these conditions, the mortality rate observed was less than 2%.
In conclusion, light intensity and substratum structural complexity are shown to be important factors in the choice of microhabitat of H. aspersa snails. The animals display a preference for light and structurally complex microhabitats, while rejecting dim light and structurally smooth microhabitats. Both factors had a similar influence on the choice of microhabitat of H. aspersa.