-
PDF
- Split View
-
Views
-
Cite
Cite
José Benchetrit, James D. McCleave, Current and historical distribution of the American eel Anguilla rostrata in the countries and territories of the Wider Caribbean, ICES Journal of Marine Science, Volume 73, Issue 1, January 2016, Pages 122–134, https://doi.org/10.1093/icesjms/fsv064
Close - Share Icon Share
Abstract
The American eel is a widely distributed, facultatively catadromous fish that is reported to range from southern Greenland to the Gulf of Mexico and Caribbean Sea. Despite such a broad distribution, our understanding of the species' biology and ecology is based on research carried out almost exclusively in Canada and the United States. As one moves south from the United States through both the Antilles and Mexico, progressively less is known about the species. Even farther south, in Central and South America, information is sufficiently scant as to raise doubt on whether or not the species occurs there at all. This study compiled available quantitative information from literature and museum records and qualitative information from other literature and personal contacts on the distribution of the American eel from Mexico, Central America, northern South America, and the Antilles, to provide the first comprehensive description of the species' historical and current distribution in the region. The results of this investigation confirm that the American eel was historically, and continues to be, widely distributed throughout the Wider Caribbean region, extending all the way to eastern Venezuela and the island of Trinidad. Furthermore, this work also addresses habitat loss and degradation, pollution, and increasing pressure from developing commercial fisheries as the major threats facing the species both locally in, and broadly throughout, the region. If effective management and conservation of the panmictic American eel is to be achieved, it is of critical importance that greater efforts be made to promote and encourage research on the species' basic ecology in the Wider Caribbean region.
Introduction
The panmictic American eel (Anguilla rostrate) (Wirth and Bernatchez, 2003; Côté et al., 2013; Pujolar, 2013), has, or once had, a range spanning at least 50° of latitude, extending from southern Greenland (Boëtius, 1985) and Labrador, through eastern Canada and the United States, Mexico, Central America, parts of northern South America, and most islands of the West Indies (Tesch, 2003). Surprisingly, there is only vague and conflicting understanding of both historical and current distribution in the tropical portion of the species' range (Cairns et al., 2014). Anecdotal information obtained by Schmidt (1909) through postal correspondence reported the American eel to be abundant in northern Mexico, absent in southern Mexico, Central America, and South America, except British Guiana, but widespread in the West Indies. In his detailed review of anguillid eels, Tesch (2003) described the southern limit of distribution as “… Mexico … and in Panama … and on the South American mainland as far as Guyana”, but he did not illustrate the species' presence in the other Central American countries, and he extended the distribution to French Guiana on the South American continent. Miller and Casselman (2014) illustrated presence only from Atlantic Canada to Central America, placing question marks along the north coast of South America. The distribution map in Aoyama et al. (2001) showed the species as absent in Central America but present in Venezuela in South America. However, Aoyama (2009) later showed a continuous distribution from Mexico through Central America and across northern South America. Various fact sheets produced for public awareness by federal, state, and private entities have even listed Brazil as the southern limit. Despite this uncertainty, it seems safe to assume that the American eel's range does indeed extend well into the tropics, unlike that of the European eel, which also has a latitudinal range of ∼50° from northern Norway to southern Morocco (Tesch, 2003).
Once considered the quintessential catadromous fish, catadromy for many eels of the genus Anguilla is now regarded as facultative rather than obligatory (McCleave and Edeline, 2009 for a review). For the American eel, for example, some individuals are strictly catadromous (Lamson et al., 2006), some remain in coastal or estuarine waters throughout continental life (Lamson et al., 2006; Thibault et al., 2007), and some move from one habitat to the other one or more times during continental life (Jessop et al., 2002; Morrison et al., 2003; Cairns et al., 2004). How the various patterns of habitat use are distributed within the region of interest here will be an important consideration in conservation efforts for the American eel as well as other anguillids. Habitat diversity is high but not uniformly distributed in the wider Caribbean, e.g. freshwater habitats are widespread in some countries and essentially absent in others. Further, distance to the spawning area in the Sargasso Sea, and hence access to habitats, varies greatly across the region.
The abundances of many temperate-zone species of Anguilla are in decline: the “endangered” American eel (Haro et al., 2000; Casselman, 2003; IUCN, 2014), the “critically endangered” European eel (Dekker, 2003a, b, 2009; IUCN, 2014), the “endangered” Japanese eel (A. japonica) (Tatsukawa, 2003; Tsukamoto et al., 2009; IUCN, 2014) and the “declining” New Zealand longfin eel (A. dieffenbachii) (Jellyman, 2009; Goodman et al., 2014). The American eel was assessed as “threatened” in Canadian waters (COSEWIC, 2012), and its status in United States waters is under review by the US Fish and Wildlife Service following a petition to list the species as “threatened” (CESAR, 2010). It is currently considered “depleted” in US waters (ASMFC, 2012).
Given the decline in abundance and recruitment of the American eel in the northern and central portions of its geographic range, it must be emphasized to gather information on its status in the southern portion of the range. The objectives are to document (1) the historical distribution, (2) the current distribution, and (3) current recruitment of juveniles in Mexico, Central America, northern South America, and the islands of the West Indies (Table 1, Figure 1).
Countries and territories considered as potentially having American eels present.
| Continental countries . | Island countries/territories . | Island countries/territories . |
|---|---|---|
| Mexico (31/16) | Aruba* (0/0) | Sint Eustatius** (0/0) |
| Guatemala (18/0) | Curaçao (present/present) | Saint Kitts* (0/0) |
| Belize (3/5) | Bonaire* (present/0) | Nevis** (0/0) |
| Honduras (133,5) | Cayman Islands** (0/0) | Barbuda** (0/0) |
| Nicaragua (36/2) | Cuba (60/>275) | Antigua** (1/0) |
| Costa Rica (26,0) | Jamaica (18/present) | Montserrat* (1/0) |
| Panama (84,6) | Bahamas** (7/0) | Guadeloupe (>148/2) |
| Colombia (4/5) | Turks and Caicos**(0/0) | Dominica (25/0) |
| Venezuela (25/3) | Haiti (128/abundant) | Martinique (abundant/≥105) |
| Guyana (0/0) | Dominican Republic (219/abundant) | Saint Lucia (1/0) |
| Republic of Suriname (0/0) | Puerto Rico (abundant/abundant) | Saint Vincent (0/0) |
| French Guiana (0/0) | British Virgin Islands** (0/0) | Grenadines** (0/presence) |
| Brazil (0/0) | US Virgin Islands* (3/8) | Barbados* (0/0) |
| Anguilla** (0/0) | Grenada (0/0) | |
| Sint Maarten/St Martin** (2/0) | Trinidad (≥66/7) | |
| Saint Barthélémy** (0/0) | Tobago* (9/0) | |
| Saba** (0/0) |
| Continental countries . | Island countries/territories . | Island countries/territories . |
|---|---|---|
| Mexico (31/16) | Aruba* (0/0) | Sint Eustatius** (0/0) |
| Guatemala (18/0) | Curaçao (present/present) | Saint Kitts* (0/0) |
| Belize (3/5) | Bonaire* (present/0) | Nevis** (0/0) |
| Honduras (133,5) | Cayman Islands** (0/0) | Barbuda** (0/0) |
| Nicaragua (36/2) | Cuba (60/>275) | Antigua** (1/0) |
| Costa Rica (26,0) | Jamaica (18/present) | Montserrat* (1/0) |
| Panama (84,6) | Bahamas** (7/0) | Guadeloupe (>148/2) |
| Colombia (4/5) | Turks and Caicos**(0/0) | Dominica (25/0) |
| Venezuela (25/3) | Haiti (128/abundant) | Martinique (abundant/≥105) |
| Guyana (0/0) | Dominican Republic (219/abundant) | Saint Lucia (1/0) |
| Republic of Suriname (0/0) | Puerto Rico (abundant/abundant) | Saint Vincent (0/0) |
| French Guiana (0/0) | British Virgin Islands** (0/0) | Grenadines** (0/presence) |
| Brazil (0/0) | US Virgin Islands* (3/8) | Barbados* (0/0) |
| Anguilla** (0/0) | Grenada (0/0) | |
| Sint Maarten/St Martin** (2/0) | Trinidad (≥66/7) | |
| Saint Barthélémy** (0/0) | Tobago* (9/0) | |
| Saba** (0/0) |
Each country listed has many rivers, except some with only a few rivers (*) or no permanent rivers (**). Numbers of records found for historical and recent distribution are presented as (xx/xx), respectively, for each country or territory. Nicaraguan records include those from the Colombian Isla de Providencia, which is offshore of Nicaragua. Trinidad and Tobago, although one country, are listed separately.
Countries and territories considered as potentially having American eels present.
| Continental countries . | Island countries/territories . | Island countries/territories . |
|---|---|---|
| Mexico (31/16) | Aruba* (0/0) | Sint Eustatius** (0/0) |
| Guatemala (18/0) | Curaçao (present/present) | Saint Kitts* (0/0) |
| Belize (3/5) | Bonaire* (present/0) | Nevis** (0/0) |
| Honduras (133,5) | Cayman Islands** (0/0) | Barbuda** (0/0) |
| Nicaragua (36/2) | Cuba (60/>275) | Antigua** (1/0) |
| Costa Rica (26,0) | Jamaica (18/present) | Montserrat* (1/0) |
| Panama (84,6) | Bahamas** (7/0) | Guadeloupe (>148/2) |
| Colombia (4/5) | Turks and Caicos**(0/0) | Dominica (25/0) |
| Venezuela (25/3) | Haiti (128/abundant) | Martinique (abundant/≥105) |
| Guyana (0/0) | Dominican Republic (219/abundant) | Saint Lucia (1/0) |
| Republic of Suriname (0/0) | Puerto Rico (abundant/abundant) | Saint Vincent (0/0) |
| French Guiana (0/0) | British Virgin Islands** (0/0) | Grenadines** (0/presence) |
| Brazil (0/0) | US Virgin Islands* (3/8) | Barbados* (0/0) |
| Anguilla** (0/0) | Grenada (0/0) | |
| Sint Maarten/St Martin** (2/0) | Trinidad (≥66/7) | |
| Saint Barthélémy** (0/0) | Tobago* (9/0) | |
| Saba** (0/0) |
| Continental countries . | Island countries/territories . | Island countries/territories . |
|---|---|---|
| Mexico (31/16) | Aruba* (0/0) | Sint Eustatius** (0/0) |
| Guatemala (18/0) | Curaçao (present/present) | Saint Kitts* (0/0) |
| Belize (3/5) | Bonaire* (present/0) | Nevis** (0/0) |
| Honduras (133,5) | Cayman Islands** (0/0) | Barbuda** (0/0) |
| Nicaragua (36/2) | Cuba (60/>275) | Antigua** (1/0) |
| Costa Rica (26,0) | Jamaica (18/present) | Montserrat* (1/0) |
| Panama (84,6) | Bahamas** (7/0) | Guadeloupe (>148/2) |
| Colombia (4/5) | Turks and Caicos**(0/0) | Dominica (25/0) |
| Venezuela (25/3) | Haiti (128/abundant) | Martinique (abundant/≥105) |
| Guyana (0/0) | Dominican Republic (219/abundant) | Saint Lucia (1/0) |
| Republic of Suriname (0/0) | Puerto Rico (abundant/abundant) | Saint Vincent (0/0) |
| French Guiana (0/0) | British Virgin Islands** (0/0) | Grenadines** (0/presence) |
| Brazil (0/0) | US Virgin Islands* (3/8) | Barbados* (0/0) |
| Anguilla** (0/0) | Grenada (0/0) | |
| Sint Maarten/St Martin** (2/0) | Trinidad (≥66/7) | |
| Saint Barthélémy** (0/0) | Tobago* (9/0) | |
| Saba** (0/0) |
Each country listed has many rivers, except some with only a few rivers (*) or no permanent rivers (**). Numbers of records found for historical and recent distribution are presented as (xx/xx), respectively, for each country or territory. Nicaraguan records include those from the Colombian Isla de Providencia, which is offshore of Nicaragua. Trinidad and Tobago, although one country, are listed separately.
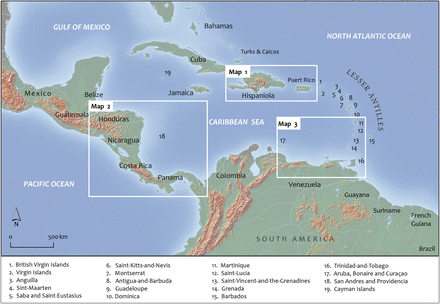
Map of the wider Caribbean region including the Gulf of Mexico, Greater and Lesser Antilles, Central America, and northern South America. Three insets, labelled Map 1—Map 3, provide subregional accounts of the distribution of a select number of specimens presented in this study. Map shape files downloaded from DIVA-GIS.
Methods
Primary, secondary, and grey literature was searched for qualitative and quantitative information on distribution and abundance of American eels in the countries and territories of interest. Online databases of museums known or suspected to house collections of American eels were searched for records of specimens. Curators at some museums were asked to provide more detail and to measure eel lengths to supplement online information. Food and Agriculture Organization of the United Nations capture fishery records was obtained for those countries where statistics existed in the online database (only Cuba, Dominican Republic, and Mexico). Personal contacts were made when an individual or entity could be identified as likely to have local knowledge of eels' presence and distribution. Records before 2000 were considered to represent historical distribution and abundance, while records from 2000 to the present to represent current distribution and abundance. This study reports records from the island of Providencia, a territory of Colombia in the Caribbean Sea but geographically closer to Nicaragua. Those records are considered along with those from Nicaragua.
It appears that systematic collecting of glass eels entering coastal and freshwaters has not been conducted, as most of the specimens documented with above methods are longer than expected for glass eels. Two other methods were used to estimate recent or current glass eel recruitment. In a few locations, recent records exhibiting a large range of lengths from one country were assumed to show that recruitment there has been a continuous process. Online advertisements or personal contacts with glass eel buyers and exporters were sought to determine whether, and to what extent, glass eel commercial fisheries exist in those areas of interest.
Results
Mexico and Central America
Historical Distribution
Mexico contains a vast area draining into the Gulf of Mexico and the Caribbean Sea (Figure 1). This represents a total of 900 000 km2 or ∼45% of the total land area draining into those two bodies of water (Martin and Meybeck, 1976). The American eel was reported to be broadly distributed throughout this area from the northern Gulf coast near Texas to the state of Quintana Roo in the Yucatan Peninsula facing the Caribbean Sea (Schmitter-Soto and Gamboa-Pérez, 1996). We documented at least 31 specimens collected between 1903 and 1989. One specimen-labelled American eel collected along Mexico's northern Gulf coast in 1989 was visually confirmed (by one of us, JB) to be a non-anguillid eel likely collected from marine waters. Similar cases of misidentification of collected specimens presented in this study are discussed in more detail below.
Of the 31 historical records presented, one was collected from a “cenote” (natural sinkhole) in the state of Quitana Roo (Yucatan Peninsula) in 1989. Most of the Yucatan Peninsula is made up of vast karstic plateau with essentially no surface freshwater flow and is instead dotted with many such sinkholes (Navarro Mendoza, 1988). Some of these are connected to the Caribbean Sea through a network of underground rivers and caves. Apparently, the American eel is present in a number of cenotes throughout the peninsula, where they are considered a nocturnal apex predator (Schmitter-Soto and Gamboa-Pérez, 1996).
There are few historical records of American eels from Belize. Three specimens were collected in 1963 from a small stream tributary of Mango Creek in the southern part of the country. These specimens ranged in length from 111 to 275 mm. To the south of Belize, a significant area (86 000 km2) of Guatemala drains into the Caribbean Sea despite its relatively short coastline (Martin and Meybeck, 1976). At least 18 specimens were collected from the country in either 1946 (17) or 1971 (1). Five of these were captured at Rio Hondo (∼60 km from the coast). The others were collected from three other rivers, at sites ∼5–10 km from the coast. No record was found of American eel from El Salvador, not surprisingly, given that the entire country drains into the Pacific Ocean (McMahan et al., 2013).
Many historical records exist of American eels captured in Honduras (Figure 2)—the most for any one country in Central America or Mexico. A total of 135 museum records were found of specimens captured between 1913 and 1995 at a number of locations spanning the breadth of the country's long Caribbean slope, including the offshore islands of Guanaja and Roatán (∼20–40 km offshore). These specimens ranged in length from 48 to 565 mm. Most of the collection locations were small streams and tributaries close to the coast. Two specimens were captured within the Rio Plátano Biosphere Reserve in the eastern part of the country. One of these was collected from Laguna de Brus, a large coastal lagoon, in 1975. Such lagoons are widespread feature in the eastern coastal lowlands of the country, extending into northern Nicaragua, and American eels are reported to occur in several others within the reserve (Cruz et al., 2002; Talavera et al., 2005).
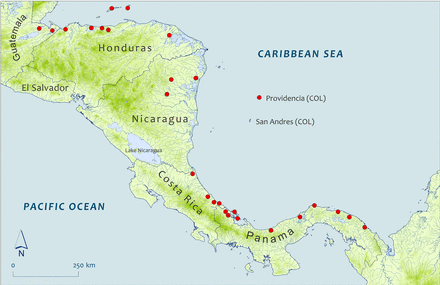
Sub Map 2: Capture/collection locations for selected specimens reported from Central America. Map shape files downloaded from DIVA-GIS.
Virtually no information regarding the American eel's historical presence in Nicaragua is available. Villa (1982) reported the species being present in the country but mentioned only that specimens had been captured as far inland as the towns of Siuna and Bonanza. Three specimens captured in the northeastern part of the country during the 1970s appear to include two of those referred to by Villa (1982) (Figure 2). Due east of Nicaragua, in the Caribbean Sea, lie the islands of San Andres and Providencia, which despite their proximity, are politically administered by Colombia. In all, 33 adult and juvenile specimens were collected from the Island of Providencia in 1968 (Figure 2). This suggests that increased sampling in the Caribbean watersheds of Nicaragua might yield a greater number of records there.
Bussing (1998) described the American eel as an uncommon species in Costa Rica's Caribbean drainages. Museum collections yielded 26 specimens caught in Costa Rica between 1964 and 1985. Most of these were collected from sites close to the coast and near Puerto Limon along the south-central Caribbean slope (Figure 2). However, six of the specimens were collected from the Tortuguero River in the north.
Despite Panama's location at the far southwestern end of the Caribbean Sea, lying a considerable distance from the spawning area in the Sargasso Sea, there are many historical records of American eels and the species appears to have been widespread there. A total of 84 records of American eels are reported here (lengths from 49 to 510 mm). Most of these are from the provinces of Bocas del Toro in the western part of the country (20 specimens), the autonomous indigenous province of Guna Yala (formerly San Blas) in the east (58 specimens) and Colón in the centre (27 specimens) (Figure 2).
Current distribution
Between January and May 2000, American eels were collected at 11 different locations in the Mexican state of Veracruz along the south Gulf of Mexico. No other information is available for these records and it was not possible to determine the total number of specimens collected. Five specimens were collected from the Soto La Marina River in Northern Mexico in 2000. Miller (2005) presented a map illustrating the species' broad distribution in the country from the northern Gulf of Mexico coast to the Yucatan Peninsula.
Six specimens recently collected in Belize included five from Stann Creek in 2006 (one of these was 345 mm standard length) and one from Spanish Creek in 2003. In far northern Guatemala, the species is reported to occur in the Laguna del Tigre National Park where “it is sometimes caught by local fishermen or those doing longterm studies” (Bestelmeyer and Alonso, 2000). A recent annotated fish species checklist for the country confirmed the species' presence in several jurisdictions in Honduras (Matamoros et al., 2009). Furthermore, five specimens were reported since the year 2000. Three were collected from Guanaja Island ∼48 km offshore in 2008 and measured 184, 187, and 264 mm long. Two other specimens were collected in the departments of Colón and Atlántida in 2010 and 2011, respectively. The American eel is reported to be captured occasionally in the watershed of the Patuca River (one of the largest rivers in Central America) where the building of a large hydroelectric dam project has been approved by the Honduran government (Esselman and Opperman, 2010).
Lake Nicaragua, >8000 km2, is the largest lake in Central America and drains into the Caribbean Sea via the San Juan River in Costa Rica. Hernández Portocarrera (2013) does not list the species among those present in Lake Nicaragua. Nevertheless, Angulo et al. (2013) claim the species to be present in the Costa Rican portion of the lake's drainage as well as all the other eight major Caribbean slope-drainages of Costa Rica. Two specimens were collected from two different locations on the island of Providencia ∼200 km east of Nicaragua in 2013 one of which was 310 mm long (C. A. Lasso, pers. comm.). Seven American eel specimens were collected between 2001 and 2013 in Panama. Three of these were captured in Changuinola River in the western part of the country in 2013 and measured 149, 161, and 355 mm long. Two specimens were captured in 2001 and 2002 (210 and 152 mm, respectively) in separate tributaries of the Coclé del Norte River in the central part of the country (province of Colón), west of the Panama Canal watershed. Another specimen (269 mm) was collected in the Cascajál River in the central part of the country in 2013. Farther to the west, in the province of Veraguas, the American eel was sampled in the Calovébora River at an unspecified date between 1997 and 2006 (Vega et al., 2006). The species apparently represents a minor source of food for the Naso Teribe, an indigenous group from western Panama and Costa Rica. In western Panama, the species has been observed in the lower parts (<20 m in altitude) of rivers (Bussing, 1998). In the Sixaloa and Telira watersheds, the species has also been captured in strong rapids at 80 m in elevation (McLarney et al., 2010a). There are also reports of the species from upper reaches (300 m in elevation) of the Bon River within La Amistad National Park—a trans-boundary national park spanning northwestern Panama and southeastern Costa Rica. The presence of the Smithsonian Tropical Research Institute, established in the Panama 1923, has likely facilitated the acquisition of substantial information on the species, both historical and recent, relative to neighbouring Costa Rica and Colombia.
South America
Historical distribution
Historical records of American eels in Colombia are sparse, largely because of lack of targeted or systematic sampling of rivers and streams there (C. A. Lasso, pers. comm.). The Río Magdalena in Colombia is the largest river flowing directly into the Caribbean Sea, draining ∼300 000 km2 of the country (Martin and Meybeck, 1976; Anonymous, 1989). Despite this, records for only four specimens are reported from Colombia, including one from the Río Gaira in 1913, two from the Río Piedras in 1978, and one from a small stream in 1988. Acero and Garzón-Ferreira (1995) reported the species to occur commonly in lower reaches of coastal drainages, but they provided no data to support the statement.
A substantially greater number of historical records were obtained from Venezuela than from Colombia. This is despite the fact that only a comparatively narrow fringe of land (150 000 km2) is available as habitat, discharging <20% of Colombia's mean annual discharge into the Caribbean Sea (47 vs. 268 km3 year−1) (Martin and Meybeck, 1976). A total of 25 American eel specimens were captured between 1954 and 1996, mostly along small drainages of the central coast and at some localities along the Paria Peninsula in the eastern part of the country's Caribbean coast (Figure 3). Acero and Garzón-Ferreira (1995) cited Cervigón (1991) as having collected a large specimen (842 mm long) but we could not obtain a copy of the latter reference to validate the claim. Reis et al. (2003), in their checklist of freshwater fish of South and Central America, state without documentation “Specimens [of the American eel] are only rarely encountered from the northern coast of South America. ”
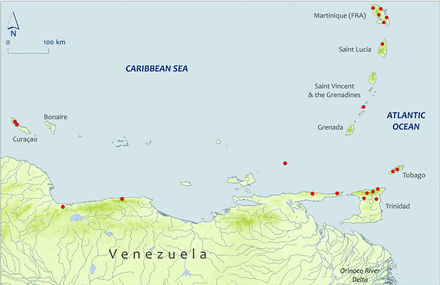
Sub Map 3: Capture/collection locations for selected specimens reported from south Lesser Antilles and Central and Eastern Venezuela. Map shape files downloaded from DIVA-GIS.
The three islands of Curaçao, Bonaire, and Aruba are overseas territories of the Netherlands and are all located on the continental shelf of South America off Venezuela. Kristensen (1971) reported capturing ∼30 specimens, most of which measured ∼300 mm, from a dam at Dokterstuin on the island of Curaçao in 1971 (Figure 3). Kristensen (1975) also reported capturing a few specimens, one measuring 280 mm, from a water catchment in Bonaire in 1975. There are no permanent freshwater streams in Curaçao, but there are many reservoirs and intermittent streams. There appears some local consumption of eels on Bonaire but no quantitative data are readily available.
Current distribution
In September 2013, five eels were caught in Quebrada Valencia, a small stream flowing into the Río Piedras in Colombia (C. A. Lasso, pers. comm.). The total length for these ranged from 260–655 mm and suggests that current recruitment is potentially continuous. DeBrot (2003) reported an unknown number of American eels (≤274 mm long) from three locations in Curaçao, one of which was hypersaline, in June 2000. The same author (pers. comm.) reported that he believed there were likely hundreds of individuals at each of the three locations. The period between late 1999 through early 2000 was abnormally rainy. Conversely, no eels were captured during the dry season of 2006 despite extensive sampling on all three islands (Hulsman et al., 2008). In Venezuela, records of three specimens are reported from separate locations spanning the breadth of the country's Caribbean coast. One eel (475 mm) was caught in 2007 at the entrance to Lake Maracaibo, a large brackish bay. A second eel (304 mm) was captured in 2008 in Los Testigos Archipelago ∼65 km north of the eastern Caribbean coast of Venezuela. The third specimen was collected in a watershed of the central coast in 2005. Historical and recent records are absent from Atlantic Ocean drainages of eastern Venezuela, including the massive Orinoco River basin despite substantial sampling effort there (Rodriguez and Lewis, 1990, 1997; Lasso et al., 2004; Rodriguez et al., 2007).
Despite several sources claiming that the American eel ranges as far south as Guyana, Suriname, French Guiana, and northern Brazil, the present investigation failed to find any record, specific mention, or other source of information that would support such claims. A recent and apparently thorough checklist of freshwater and estuarine fish in French Guiana does not include the American eel (Le Bail et al., 2012). The species is not reported to be present in the Orinoco River drainage in eastern Venezuela (Rodriguez and Lewis, 1990, 1997; Lasso et al., 2004; Rodriguez et al., 2007) and is not present in rivers of the Guiana Shield (Vari et al., 2009). Based on information presented here, the true southern limit of distribution of the species is, and probably always was, in the immediate vicinity of the island of Trinidad and Caribbean Sea drainages of eastern Venezuela.
West Indies (Bahamas Archipelago, Greater Antilles, and Lesser Antilles)
Historical distribution
The distribution and abundance of American eels seem to have been approximately proportional to the amount of riverine freshwater habitat present in islands of the West Indies. Museum records and publications are scarce or absent for many of the countries, understandable for those without permanent rivers (Table 1). Absence or scarcity of records for some islands with permanent rivers probably reflect lack of sampling rather than lack of eels such as is true for Saint Lucia, Saint Vincent, and Grenada.
Working from west to east in the West Indies, at least 60 historical records are available for Cuba, including ∼15 from the 1860s and 1870s. Most specimens were from the northeast and southeast and a few were from the northwest of Cuba. Lengths of those measured ranged from 80 to 770 mm long. At least 21 of the 60 records were of eels collected in the late 1990s in Holguin Province for an artificial maturation experiment (Canino et al., 2000). A limited commercial fishery for glass eels developed experimentally in 1974–1977 in five northeastern Cuban rivers (Fernández and Vázquez, 1978) indicating substantial historical abundance (Table 2).
Numbers and biomass of American eels caught per 100 linear meters of electrofishing in rivers of Martinique in 1994 (from Lim et al., 1997).
| River . | March–April . | September–October . |
|---|---|---|
| Grande Rivière | 5, <1 | 4, 1 |
| Rivière Capot | 2, 1 | 5, 2 |
| Rivière du Lorrain | 18, 5 | 59, 9 |
| Rivière Roxelane | 2, 1 | 2, 1 |
| Grand Rivière Pilote | 3, <1 | 0, 0 |
| River . | March–April . | September–October . |
|---|---|---|
| Grande Rivière | 5, <1 | 4, 1 |
| Rivière Capot | 2, 1 | 5, 2 |
| Rivière du Lorrain | 18, 5 | 59, 9 |
| Rivière Roxelane | 2, 1 | 2, 1 |
| Grand Rivière Pilote | 3, <1 | 0, 0 |
Data expressed as number, biomass (kg).
Numbers and biomass of American eels caught per 100 linear meters of electrofishing in rivers of Martinique in 1994 (from Lim et al., 1997).
| River . | March–April . | September–October . |
|---|---|---|
| Grande Rivière | 5, <1 | 4, 1 |
| Rivière Capot | 2, 1 | 5, 2 |
| Rivière du Lorrain | 18, 5 | 59, 9 |
| Rivière Roxelane | 2, 1 | 2, 1 |
| Grand Rivière Pilote | 3, <1 | 0, 0 |
| River . | March–April . | September–October . |
|---|---|---|
| Grande Rivière | 5, <1 | 4, 1 |
| Rivière Capot | 2, 1 | 5, 2 |
| Rivière du Lorrain | 18, 5 | 59, 9 |
| Rivière Roxelane | 2, 1 | 2, 1 |
| Grand Rivière Pilote | 3, <1 | 0, 0 |
Data expressed as number, biomass (kg).
A few museum specimens of juvenile and adult American eels were collected in waters of Jamaica mostly in the eastern quarter of the country; 18 eels ranged from 51 to 516 mm long. Most of the museum records of American eels in the Bahamas were of leptocephali caught at sea, and not juveniles or adults. However, eight eels, 177–540 mm long, could be confirmed as juveniles.
In December 1995, 115 elvers of the American eel were caught at unspecified locations in Haiti on the island of Hispaniola (Wang and Tzeng, 1998). These ranged from 41 to 51 mm long and averaged 47.8 mm long. Thirteen larger specimens were recorded from museums (134–614 mm long, n = 9) mostly from the Gulf of Gonave area in the west of Haiti (Figure 4). Historical records are abundant for the Dominican Republic. Grassia and Rizzotti (1989) reported 181 eels from the Masacre and Yaque del Norte Rivers on the north coast and two from the Ozama River on the south coast (Figure 4). These eels ranged in length from 190 to 550 mm. Thirty-six museum specimens came about half from a stream on the south coast and about half from streams on the north coast. All but two were measured, ranging from 49 to 398 mm long.
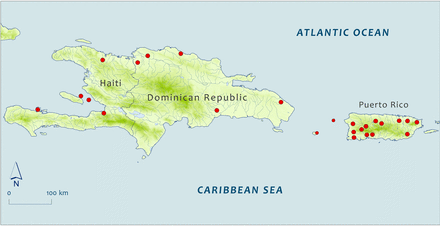
Sub Map 1: Capture/collection locations of selected specimens reported from the islands of Hispaniola and Puerto Rico in the Greater Antilles. Map shape files downloaded from DIVA-GIS.
Before 2000, many American eels were collected in Puerto Rico (deposited in museums or reported in Smith et al., 2008). Smith et al. (2008) reported that a gillnet survey in 1977 in the Río Esperitu Santo estuary in the northeast of Puerto Rico ranked American eels the tenth in abundance of 60 species captured, 2.79% of the total catch. Museum collections yielded 117 specimens, and where location was known, 67 were from north-coast streams, 21 from west-coast rivers, two from south-coast rivers, and one each from Desecheo Island (285 mm) and Mona Island (250 mm) in Mona Passage between Puerto Rico and the Dominican Republic (Figure 4). The north-coast lead is in part be due to distribution of sampling effort but also may be in part due to proximity to the spawning area. Mainland specimens ranged from 47 to 429 mm long plus a supposed 688-mm long specimen from the San Juan market in 1899.
Gillet (1983) reported capturing 115 eels at 10 different sites on the island of Basse-Terre in Guadeloupe between 1978 and 1980. The distribution and numbers of individuals caught at 14 of 51 sampling sites on Basse-Terre island in March–April, 1995 were not specified (Fièvet et al., 2001a), but there were 17 eels caught in the Grand-Carbet River between April 1991 and January 1998 (Fièvet et al., 2001b) and two caught in the Bannier River in March 1997 (Fièvet et al., 1999), both rivers in southeast Basse-Terre. There are no permanent rivers on Grande-Terre, the other island of Guadeloupe.
In March and October 1994, American eels were caught in three river basins on the north coast and one river basin on the south coast of Martinique but not in three river basins on the west coast (Figure 3) (Lim et al., 1997). Where present, 2–59 eels and <1–9 kg per 100 linear meters of electrofishing were reported (Table 2). Twenty-one American eels were caught in July 1977, in three small streams in Dominica.
Kenny (1995) and Phillip (1998) both collected fish widely in Trinidad and a few museum specimens of American eels were also located, all in the northern part of Trinidad. Their records plus the museum specimens document common occurrence (>66 records) in five rivers flowing north and four flowing south from the mountains of the Northern Range; two rivers join and drain into the Caribbean Sea in the northwest, and two join and drain into the Atlantic Ocean in the northeast (Figure 5). Phillip (1998) also reported nine specimens from four rivers in Tobago.
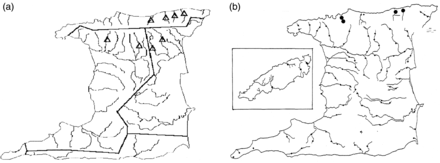
American eel records (a) from Trinidad figured by Kenny (1995) and (b) from Trinidad and Tobago figured by Phillip (1998). Both authors sampled throughout Trinidad. Tobago is located ∼38 km northeast of Trinidad; Tobago is inset in (b) for simplicity.
Current distribution
Beginning in 2000, systematic collecting has occurred in the Commonwealth of Puerto Rico (museum specimens; Kwak et al., 2007; Cooney and Kwak, 2013), in St John in the US Virgin Islands (Loftus, 2003), in Martinique (Asconit Consultants, pers. comm.), and to a small degree in north-coast rivers in Trinidad in 2014 (D. A. T. Phillip, pers. Comm.). A few records are also available for Cuba. American eels were taken in Cuba for experimental purposes in the early 2000s. Four were caught in June 2005 in the Sagua la Grande River, ranging in length from 650 to 770 mm (De la Rosa et al., 2009). An estimated 270 elvers (mean weight = 0.63 g) were taken in undisclosed location(s) probably in the early 2000s for a nutritional experiment (Fraga et al., 2003).
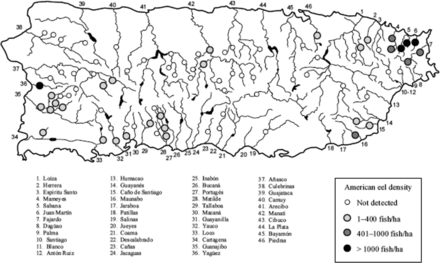
Eels were described as widespread in Puerto Rico based on past records (Neal et al., 2009) and sampling in the mid to late 2000s (present at 32 of 81 stations, Figure 6, Kwak et al., 2007; present at 57 of 118 stations likely including the previous 81 stations, Cooney and Kwak, 2013), but were actually clustered in the southwest and northeast portions of the commonwealth and were not detected in many drainages (Figure 6) (Kwak et al., 2007; Cooney and Kwak, 2013). American eels ranked 14 of 25 species in mean density at 462 eels per hectare (Kwak et al. (2007). Compared with means for all 81 sampling sites, eels were found at lower elevations (mean 53.47 vs. 166.55 m), at lower stream gradients (1.42 vs. 2.45%), and closer to the mouths of rivers (16.35 vs. 27.96 km).
Occurrence and density of American eel among 81 sites sampled during 2006–2007 spanning 34 of 46 drainage basins in Puerto Rico. From Kwak et al. (2007) permission requested.
Eels were quite rare on St John in the US Virgin Islands between 2001 and 2003, six eels being recorded from only three of 67 sites sampled, probably because the guts (streams) do not run all year anymore (Loftus, 2003). American eels were present in all 19 sites in all 15 river drainages in Martinique sampled between 2006 and 2010 (Asconit Consultants, pers. comm.). About 73% of the eels were caught in rivers sampled in Atlantic Ocean drainages and the rest from Caribbean Sea drainages. Seven individuals were caught in February–March, 2014, in five rivers on the north coast of Trinidad (D. A. T. Phillip, pers. comm.).
There is no evidence that American eels inhabit high-salinity mangrove lagoons or sea grass beds, although negative evidence may not be conclusive. At selected locations, intensive sampling of mangroves (Bouchereau et al., 2008) and seagrass beds (Bouchon-Navaro et al., 2006; Kopp et al., 2010) in Guadeloupe and mangroves (Louis et al., 1992) and seagrass beds (Bouchon-Navaro et al., 1992,, 2006) in Martinique caught no American eels.
Recruitment in the region
To the best of our knowledge, there are no specific surveys of glass eel recruitment in the West Indies. Recruitment in the Holguin Province in northeast Cuba in the mid-1970s was sufficient to allow a modest commercial fishery for glass eels in five rivers in the Gibara and Bariay drainage basins (Fernández and Vázquez, 1978). Total captures during the fishing season (October to February) were 6930, 3622, and 380 kg in 1974, 1976, and 1977, respectively (Table 3). According to D. Sprague (pers. comm.), Cuba has “enormous quantities of glass eel” migrating into rivers in Cuba. There apparently is a current glass eel fishery of limited extent in Cuba, operating for export to Asia (R. Bushey, pers. comm.; M. Feigenbaum, pers. comm.).
Commercial fishery for American eel glass eels in Holguin Province, Cuba, in the mid-1970s.
| River . | Numbers of fishers and catch (kg) by river . | Total . | ||||
|---|---|---|---|---|---|---|
| Gibara . | Cacoyuguín . | Bariay . | Guadajaney . | Yabazón . | ||
| Number of fishers | 15 | 13 | 17 | 2 | 5 | 52 |
| Catch (kg) | 165 | 153 | 52 | 7 | 3 | 380 |
| Catch (kg) by month and year | ||||||
| Month | October | November | December | January | February | |
| 1974 | 1,840 | 3,154 | 1,665 | 272 | – | 6,931 |
| 1976 | 222 | 3,038 | 206 | 156 | – | 3,622 |
| 1977 | 15 | 102 | 225 | 8 | 30 | 380 |
| River . | Numbers of fishers and catch (kg) by river . | Total . | ||||
|---|---|---|---|---|---|---|
| Gibara . | Cacoyuguín . | Bariay . | Guadajaney . | Yabazón . | ||
| Number of fishers | 15 | 13 | 17 | 2 | 5 | 52 |
| Catch (kg) | 165 | 153 | 52 | 7 | 3 | 380 |
| Catch (kg) by month and year | ||||||
| Month | October | November | December | January | February | |
| 1974 | 1,840 | 3,154 | 1,665 | 272 | – | 6,931 |
| 1976 | 222 | 3,038 | 206 | 156 | – | 3,622 |
| 1977 | 15 | 102 | 225 | 8 | 30 | 380 |
Commercial fishery for American eel glass eels in Holguin Province, Cuba, in the mid-1970s.
| River . | Numbers of fishers and catch (kg) by river . | Total . | ||||
|---|---|---|---|---|---|---|
| Gibara . | Cacoyuguín . | Bariay . | Guadajaney . | Yabazón . | ||
| Number of fishers | 15 | 13 | 17 | 2 | 5 | 52 |
| Catch (kg) | 165 | 153 | 52 | 7 | 3 | 380 |
| Catch (kg) by month and year | ||||||
| Month | October | November | December | January | February | |
| 1974 | 1,840 | 3,154 | 1,665 | 272 | – | 6,931 |
| 1976 | 222 | 3,038 | 206 | 156 | – | 3,622 |
| 1977 | 15 | 102 | 225 | 8 | 30 | 380 |
| River . | Numbers of fishers and catch (kg) by river . | Total . | ||||
|---|---|---|---|---|---|---|
| Gibara . | Cacoyuguín . | Bariay . | Guadajaney . | Yabazón . | ||
| Number of fishers | 15 | 13 | 17 | 2 | 5 | 52 |
| Catch (kg) | 165 | 153 | 52 | 7 | 3 | 380 |
| Catch (kg) by month and year | ||||||
| Month | October | November | December | January | February | |
| 1974 | 1,840 | 3,154 | 1,665 | 272 | – | 6,931 |
| 1976 | 222 | 3,038 | 206 | 156 | – | 3,622 |
| 1977 | 15 | 102 | 225 | 8 | 30 | 380 |
Evidence that recruitment occurs regularly in Jamaica came from a newspaper article that appeared in the Jamaica Gleaner, 25 November 2005, “Migrating Atlantic eels, which make their annual run from the Sargasso Sea … to the Great River [Jamaica] have sparked interest among local conservationists …” (http://jamaica-gleaner.com/gleaner/20051125/news/news9.html; accessed 17 February 2015). The newspaper erroneously described them as Anguilla anguilla. More recently, R. Bushey (pers. comm.) is playing an advisory role in conjunction with the Jamaican fisheries agency and local fishers in 3-year exploratory glass eel fishery. In 2014 after a rain event, fishers caught 30–40 kg in a night.
Wang and Tzeng (1998) collected 115 glass eels by dipnet in December, 1995, at an undisclosed location in Haiti. There are current glass eel fisheries in both Haiti and the Dominican Republic indicating substantial annual recruitment. Anecdotal information suggests perhaps 4 metric tons per year for each country (W. Jackson, pers. comm.) or 5–6 metric tons per year total for Hispaniola (M. Feigenbaum, pers. comm.). The Dominican newspaper El Nacional reported in May 2013 that the Dominican Republic's Consejo Dominicano de Pesca y Acuicultura (CODOPESCA) released 50 000 juvenile American eels (presumably glass eels) into the Río Yásica on the north coast to encourage production of the species (http://elnacional.com.do/agricultura-libera-50-mil-anguilla-rostrata, accessed 17 February 015). Dominican glass eel fishing companies were urged to return part of their catch to the rivers where they fished to preserve fishing for the future. CODOPESCA licensed nine companies to fish for glass eels during the 2014–2015 season.
Seven of 94 eels measured on Martinique between 2006 and 2011 were <150 mm long (Asconit Consultants, pers. comm.), indicating some recruitment during the 2000s. W. Jackson (pers. comm.) and a Haitian colleague travelled extensively talking to local people in the Caribbean islands as far as Martinique in 2004, finding glass eels wherever there were rivers.
Discussion
There are limitations to the approaches used here, the results of which are findings that vary both qualitatively and quantitatively from country to country. Systematic surveys of freshwater and diadromous fish range from excellent for Puerto Rico (Kwak et al., 2007; Cooney and Kwak, 2013), Guadeloupe (Fièvet et al., 2001a, b), and Martinique (Lim et al., 1997; Asconit Consultants, pers. comm.), to very good for Trinidad and Tobago (Phillip, 1998) and Panama, to poor or absent for most of Central America, Colombia, and Venezuela. The authors accepted museum records at face value unless a curator informed us of a misidentification listed in a database (one misidentification) or when some Mexican museum specimens were examined by one of us (one misidentification). Some collection records did not include the collection date, but those records were assigned to historical or current distribution numbers based on ancillary evidence. It is unlikely that conclusions presented would change because of an occasional misidentification or incorrect distinction between historical and current distribution.
Nevertheless, this study has shown that the American eel historically was, and recently is, distributed widely in the islands of the West Indies where freshwater rivers and lakes occur, and throughout the Caribbean Sea drainages of Mexico, Central America, and Colombia and western Venezuela in South America. They are apparently absent in all Atlantic Ocean drainages of South America, including the massive Oronoco River basin draining most of Venezuela and eastern Colombia (Rodríguez and Lewis, 1990, 1997; Lasso et al., 2004; Rodríguez et al., 2007), as well as those in Guyana, Suriname, French Guiana, and northern Brazil (Vari et al., 2009; Le Bail et al., 2012). The apparent absence of eels in the southern two-thirds of Trinidad matches their absence in the Orinoco River, the mouth of which is only a few kilometres to the south. The northwest-flowing North Brazil/Guyana/Caribbean Current (Johns et al., 1998, 2002; Artigas et al., 2003) would preclude American eel leptocephali reaching these southern areas. Albert and Reis (2011) listed the species as present in the Amazon Superbasin based on papers by Minegishi et al. (2005) and Aoyama (2009). However, the papers were misinterpreted. Both Aoyama et al. (2001) and Minegishi et al. (2005) used the exact same sentence “… anguillid eels are absent [emphasis added] along the east coast of South America, despite the existence of the warm Brazil Current. ”Aoyama (2009) does not mention the word Brazil. All three papers show world maps of Anguilla distribution; none shows Brazil in the distribution of Anguilla rostrata.
It is likely that the current abundance of American eels in the southern regions is lower tha historically, due largely to loss of habitat, as has been documented for Canada (MacGregor et al., 2009; Cairns et al., 2014; Pratt, 2014) and the United States (Busch et al., 1998; Machut et al., 2007; Greene et al., 2009). Pringle et al. (2000a, b) wrote of rapid and continuing degradation of river systems in the tropics due to first, human population growth and urbanization leading especially to increased discharge of untreated sewage; second, expanding agriculture leading to discharge of fertilizers, pesticides and herbicides into rivers and water extraction for irrigation; third, multiple effects of deforestation; and fourth, damming for hydropower generation. March et al. (2003) provided a general review of the problems associated with dams and water extraction on migratory species of invertebrates and fish on tropical islands. Dams can extirpate migratory species from upper reaches, and where blockage is incomplete, can cause mortality of downstream-moving individuals. Tropical islands in the West Indies may be especially vulnerable to effects of dams and water extraction, as the aquatic macrofauna are dominated by diadromous fish and invertebrates (Fièvet et al., 2001b).
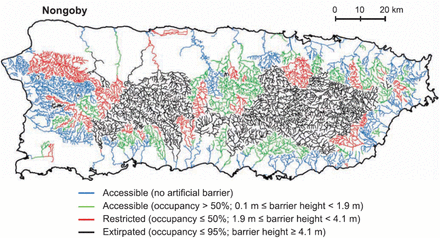
The non-goby assemblage of fish, including the American eel, is extirpated or with restricted occupancy in large areas of Puerto Rican river drainages (Figure 7) (Cooney and Kwak, 2013). Cooney and Kwak (2013) documented the existence of 335 dams in all 46 drainage basins. Water extraction for agriculture and other uses has completely dewatered many sections of streams. Dams averaging 0.9 and 3.0 m high block ∼50 and 95% of American eel migration, respectively, in Puerto Rico (Cooney and Kwak, 2013). Using Puerto Rico as a case study, Hemphill and García (2002) listed culverts, erosion, industrial and domestic pollution, dredging, and stream channelization, in addition to dams and water extraction, as all contributing to deterioration of aquatic habitat and migratory corridors.
Accessible, restricted, and extirpated river reaches in Puerto Rico for native diadromous non-goby fish, including the American eel, by artificial in-stream barriers. From Cooney and Kwak (2013) with permission.
The construction of a low-head dam and a water extraction scheme on a fast-flowing river in southeastern Guadeloupe caused water depths and discharge to decrease, the latter by >50%. The relative abundance of the guild of secondary consumer fish, all diadromous including the American eel, was significantly lower at the three below-dam sampling sites as a result (Fièvet et al., 2001b). Kenny (1995) attributed the probable-extirpation of American eels from the Caroni River drainage on the island of Trinidad primarily to industrial and domestic pollution. Phillip (1998) characterized sampling sites in Trinidad as polluted (13), perturbed (42), and pristine (14) and in Tobago as perturbed (2) and pristine (9). She attributed pollution and perturbation to deforestation, domestic sewage and dumping, agricultural pesticides and wastes, oil production and industrial waste discharge, and quarrying. Both Phillip (1998) and apparently Kenny (1995) collected American eels only at locations described as pristine.
Habitat loss is not just a problem for American eels and other fish on tropical islands; it is widespread in the continental countries of interest here. Habitat degradation and loss is extensive in river systems and the coastal areas of Mexico. Inland, contributions to these losses have come from water and oil extraction, river channelization, domestic and industrial pollution, mining, agriculture and tourism related activities (Soto-Galera et al., 1998; López-López et al., 2009). In the coastal areas along the Gulf of Mexico, many factors are responsible, but oil drilling, refining, transport, waste discharge, and port activities are leading contributors (Ortiz-Lozano et al. (2005).
Threats of habitat loss extend into the future, a case in point being the proposal to construct many hydroelectric dams in five major Caribbean Sea draining river watershed partly in the La Amistad World Heritage Site (PILA) and adjacent indigenous peoples' reserves in Costa Rica and Panama (McLarney et al., 2010a, b). Most of the remaining untapped hydropower is in the tropics (Anderson et al., 2006). For example, there are currently no dams along the Rio Urem in the Rio Sixaola watershed. However, environmental impacts from proposed dam constructions are thought to be “potentially catastrophic” (McLarney et al., 2010b). Likewise, open pit mining activity is minimal in the same area, but the impacts from proposed developments are “potentially very severe” (McLarney et al., 2010b). These threats add to lesser current habitat degradation from deforestation, livestock incursion, agrichemicals, domestic waste, etc. (McLarney et al., 2010a, b). Elsewhere in the region, Esselman and Opperman (2010) also stressed the difficulty in reconciling the longer term impacts on migratory fish potentially posed by the hydroelectric dam project along the Patuca River in eastern Honduras. Damming is especially detrimental to the functioning of freshwater ecosystems in Central America, as is true in the West Indian islands, because such a large proportion of the fish and invertebrate macrofauna are diadromous or amphidromous.
The development of new commercial fisheries for glass eels should be discouraged, given the extent of, and potential for, habitat loss throughout the American eel's range, and particularly in the tropics, especially in the tropics. In addition to threat of habitat loss, there is a clear lack of scientific studies on glass eel recruitment in the southern portion of the range notwithstanding limited, and often anecdotal, information presented for Cuba, the Dominican Republic and Haiti. Field surveys of American eel abundance and size distribution together with surveys of glass eel recruitment in several countries in the region should be conducted before additional fishing pressure is placed on the species. Given the region's proximity to the Sargasso Sea, such information would be of considerable use to resource managers and decision makers aiming to manage this broad-ranging panmictic species.
Acknowledgements
Among the many museum curators that readily provided additional data and information regarding relevant specimens housed in their respective collections, we thank, in no particular order, Barbara Brown, Adam Cohen, Oliver Crimmen, Rick Feeney, James Gilliam, Karsten Hartel, Erling Holm, William Loftus, Susan Mochel, Douglas Rodriguez, William and Myrna López, Robert Robbins, David Smith, Wayne Starns and Maridith Gatens, Chris Taylor, Noel Alfonso and Sylvie Laframboise, and the staff from the Smithsonian Tropical Research Institute's Neotropical Freshwater Fish collection. Catherine Desrosiers (ASCONIT Consultants), Dawn Phillip, and Carlos Andrés Lasso were extremely helpful in providing information on American eels, respectively in Martinique, Trinidad and Tobago, and Colombia. The authors are particularly grateful for their efforts to provide data from the American eels collected during their field efforts. Finally, we thank Amanda Moeser for contributing to the compilation of data and information from several countries and territories in our region of interest.
References
Author notes
Both authors contributed equally to this work.
Handling editor: Caroline Durif